Why the FDA is tightening access to COVID boosters this fall
Why the FDA is tightening access to COVID boosters this fall
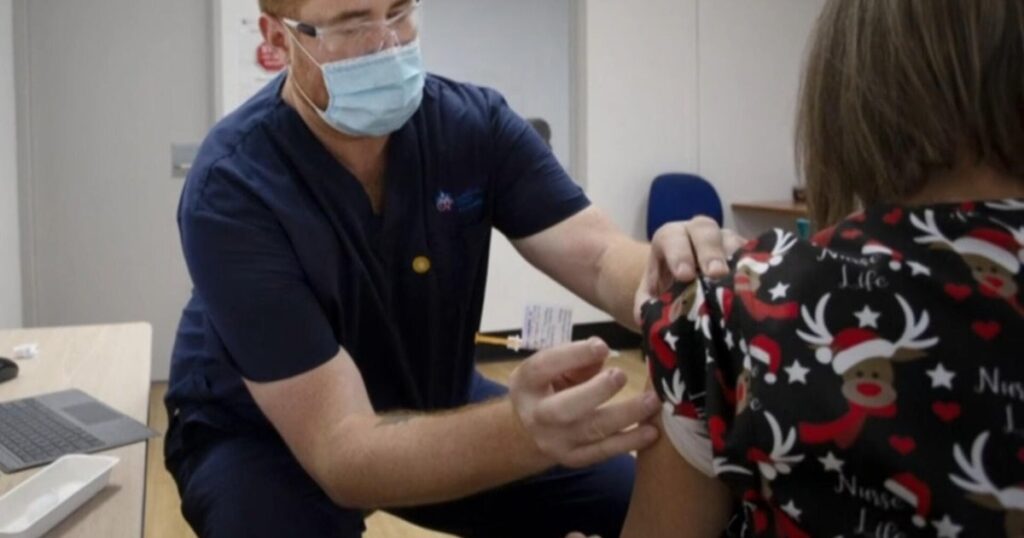
The Food and Drug Administration says it has decided to continue approving COVID-19 vaccine updates for seniors and others at higher risk of severe disease, but will require vaccine makers to conduct major new clinical trials before approving them for wider use. Dr. Celine Gounder breaks it down.
Read the full article on CBS US
Truth Analysis
Analysis Summary:
The article is mostly accurate, reflecting the FDA's decision to tighten access to COVID boosters. The main claim regarding the FDA's decision is supported by multiple sources. However, there's a slight slant towards framing the decision as a restriction, and some details lack sufficient context, contributing to a moderate bias.
Detailed Analysis:
- Claim:** The FDA has decided to continue approving COVID-19 vaccine updates for seniors and others at higher risk of severe disease.
- Verification Source #1: Supports this claim, stating the FDA will OK updated vaccines for high-risk groups.
- Verification Source #2: Supports this claim by mentioning the FDA's new requirements for access to yearly COVID shots.
- Verification Source #3: Supports this claim by stating that many Americans without underlying conditions may not have access to updated shots.
- Claim:** The FDA will require vaccine makers to conduct major new clinical trials before approving them for wider use.
- Verification Source #1: Supports this claim, mentioning the FDA's requirement for clinical trials.
- Verification Source #2: Supports this claim by mentioning the FDA laid out new requirements.
- Claim:** The FDA is tightening access to COVID boosters this fall.
- Verification Source #1: Supports this claim, mentioning that other developed countries have already limited annual COVID-19 vaccine boosters to only older adults and those with certain conditions.
- Verification Source #3: Supports this claim, stating the FDA is tightening rules.
Supporting Evidence/Contradictions:
- Verification Source #1: "They also pointed to other developed countries that have already limited annual COVID-19 vaccine boosters to only older adults and those with…" This supports the claim that the FDA is tightening access, aligning with practices in other countries.
- Verification Source #2: "The FDA laid out new requirements for access to yearly COVID shots, saying they'd…" This supports the claim that the FDA is tightening requirements for COVID vaccines.
- Verification Source #3: "The decision means many Americans without underlying conditions may not have access to updated shots." This supports the claim that access to updated shots is being limited.
- Verification Source #4: While this source discusses the benefits of vaccines, it doesn't directly address the tightening of access, so it neither supports nor contradicts the main claims.
- Verification Source #5: This source is a timeline from 2021 and discusses expedited trials for boosters, which is related but doesn't directly address the current tightening of access.